"In constrast to conventional biocidal active substances, the temporal concentration of ONOOH is precisely controlled in the Sporosan®-process so that the active substance is released just-in-time and without residual toxicity."
Dr. Jörn Winter
CEO and Founder

Special features of peroxynitrous acid (ONOOH)
Peroxynitrous acid (ONOOH) is an unusual biocidal active, as it features a lifetime of only 1 second at room temperature [1]. However, this property, together with its unrivalled biocidal activity, allows for completely new processes in high-level disinfection.
Conventional biocidal active substances usually depend either on a high concentration of the active substance itself or on long exposure times in order to achieve the desired biocidal effect. Since the effect of such active substances follows a dose-response relationship such as Haber's rule (effect = concentration x exposure time), we can typically choose between the following:
1. high concentration leading to poor material compatibility and fast antimicrobial activity or2. low concentration leading to good material compatibility yet slow antimicrobial activity.
While Haber's rule is generally true for the biocidal activity of Sporosan®, it comes with a twist: Since the active ONOOH decomposes directly from its reactants nitrite and hydrogen peroxide after its generation, the concentration of ONOOH is strongly time-dependent and can be controlled, for example, by means of the pH value.
The following graph shows two procedures that are optimized for an exposure time of 30 seconds and 10 minutes. Both processes have the same biocidal activity. As shown, ONOOH is only released during the exposure time, so the residual toxicity drops to zero as soon as the respective process has ended. This means that both processes also cause the same low oxidative stress on materials and skin, albeit featuring very different exposure times.
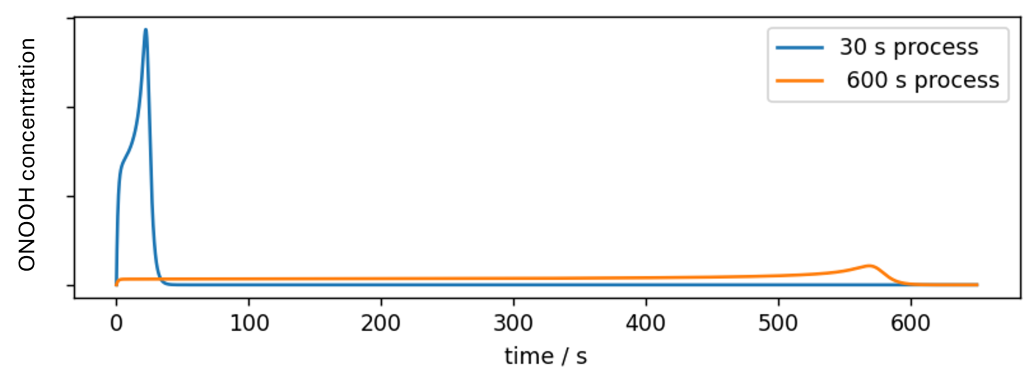
While even longer process times can be achieved, the Sporosan®-process generally outclasses other biocidal active substances when short exposure times are required. This can be taken to the extreme, as shown in the following figure, in which a sporicidal process of 5 seconds is realized.

Delayed release of the active substance
Such extremely fast processes have the disadvantage that they are prone to handling errors, e.g. the disinfectant can not be used after the intended exposure time as the biocidal active substance has completely disappeared. Hence, such formulations are only of interest for automated disinfection processes, e.g. in washer-disinfectors.
However, to obtain systems that are applicable for manual disinfection of surfaces, a retarding agent can be added to the mixture, which evaporates as soon as the disinfectant is spread onto a surface. While the retarding agent completely inhibits the reaction of hydrogen peroxyde and nitrite on the reaction time scale, the reaction starts as soon as a significant amount of the retarding agent has evaporated. The typical temporal development of ONOOH during the application of such a disinfection system on a surface is shown in the following figure.
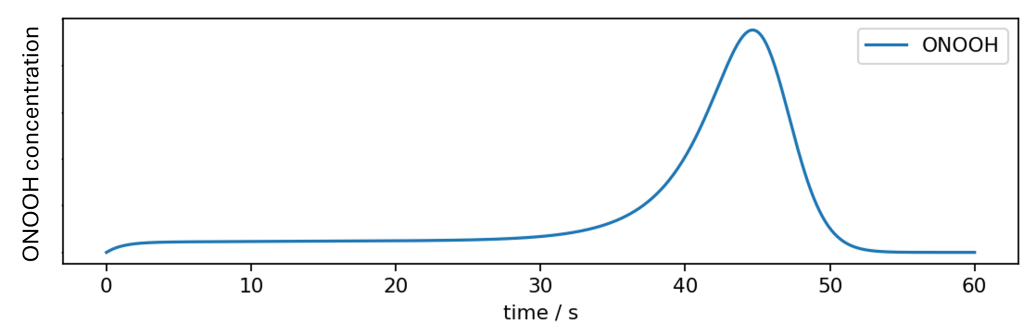
Such processes come very close to the ideal temporal profile of biocidal activity in surface disinfection:
- No activity while the disinfectant is being distributed,
- High activity when the disinfectant evaporates,
- Complete broad-spectrum disinfection without any toxic residues once the disinfectant has evaporated.
Chemistry of the Sporosan®-process
The Sporosan®-process is based on the generation of ONOOH from nitrite and hydrogen peroxide under acidic conditions. This combination is a novel antimicrobial agent that as of now has not been used in disinfection products. ONOOH generated in the Sporosan® process can only be formed under acidic conditions according to the following reactions [2]:
\[ \begin{aligned} \text{H}_2\text{O}_2 + \text{H}_3\text{O}^+ &\rightleftharpoons \text{H}_3\text{O}_2^+ + \text{H}_2\text{O} \quad &\text{(R1)} \\ \text{N}\text{O}_2^- + \text{H}_3\text{O}^+ &\rightleftharpoons \text{H}\text{NO}_2 + \text{H}_2\text{O} \quad &\text{(R2)} \\ \text{H}_3\text{O}_2^+ + \text{HNO}_2 &\rightarrow \text{ONOOH} + \text{H}_3\text{O}^+ \quad &\text{(R3)} \end{aligned} \]
Reactions (R1) to (R3) can be summarized in the net reaction equation
\[ \begin{aligned} \text{HNO}^{\text{tot}}_2+\text{H}_2\text{O}_2 &\rightarrow \text{ONOOH}+ \text{H}_2\text{O} \quad &\text{(R4)}\end{aligned} \]
where \(\text{HNO}^{\text{tot}}_2\) is the sum of \(\text{NO}_2^-\) and the corresponding conjugated acid \(\text{HNO}_2\).
Due to the strong pH dependency of the R4 reaction, the Sporosan®-process always requires an acidic pH value - typically between 2 and 3.5 - in order to achieve process times ranging from a few seconds to several minutes.
The lifetime of peroxinitrous acid is 1 second. After this time, ONOOH partially isomerizes to nitric acid (HNO3) or decomposes to hydroxyl radicals and nitrogen dioxide [3]:
\[ \begin{aligned} \text{ONOOH} &\rightarrow \text{NO}_2 + \text{OH}\quad &\text{(R5)} \\ \text{ONOOH}&\rightarrow \text{NO}_3^- + \text{H}^+\quad &\text{(R6)} \end{aligned} \]
Since NO₂ is highly soluble in water and the pKa value of HNO₃ is -1.3, which is far below the pH values found in Sporosan® application solutions, only a minor proportion can outgas from the solution, while most of the NO₂ is converted directly to NO₃- according to
\[ \begin{aligned} \text{NO}_2+\text{OH} &\rightarrow \text{NO}_3^- + \text{H}^+\quad &\text{(R7)} \end{aligned} \]
The entire process can therefore be described as the conversion of NO₂- into NO₃- with ONOOH as an intermediate product.
Differentiation between endogenous ONOO⁻ and ONOOH generated from NO₂⁻ und H₂O₂
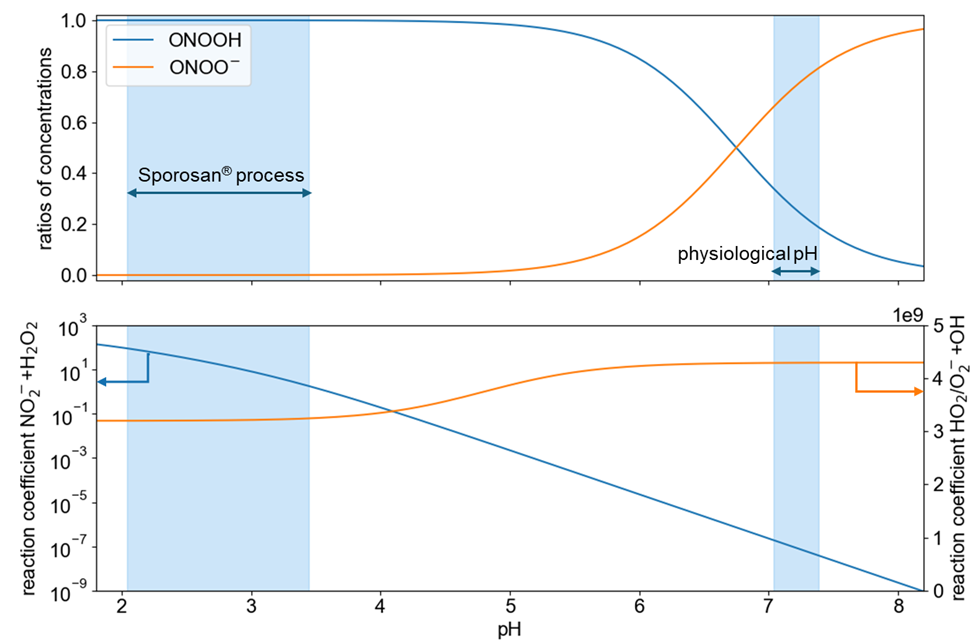
The current literature mainly focuses on endogenous peroxynitrite (ONOO⁻). This conjugated ONOOH anion (pKs 6.8 [4]) is widely recognized as an antimicrobial species secreted by macrophages as part of the innate immune response [5]. At a physiological pH value of 7.0 to 7.4, for example, ONOO⁻ is the dominant species, whereas in the pH range of the Sporosan® process, where a pH value between 2.0 and 3.5 is used, ONOOH is clearly the dominant species (see graph below, top). While ONOO⁻ is theoretically stable at alkaline pH, it reacts rapidly with CO₂, which conveys its further biological effect and leads to an effective lifetime of 1-2 ms [6] at physiological CO₂ concentrations. In the body, ONOO⁻ is formed from superoxide (O₂⁻) or hydroperoxyl (HO₂) and nitric oxide(NO). This process is largely independent of the pH value, as shown in the following figure (bottom).
In contrast, the R4 reaction used in the Sporosan® process is strongly pH-dependent, which makes the generation of ONOOH at physiological pH practically impossible:
A typical Sporosan® process, which normally takes about 15 seconds, would take about 3 years at a pH value of 7. Therefore, a direct systemic effect of ONOOH generated by Sporosan® is neither to be expected nor experimentally observed.
Literature:
[1] C. Molina, R. Kissner, and W. H. Koppenol, "Decomposition kinetics of peroxynitrite: influence of pH and buffer," Dalton Transactions, vol. 42, no. 27, p. 9898, 2013. doi: 10.1039/c3dt50945a. [Online]. Available: http://xlink.rsc.org/?DOI=c3dt50945a
[2] D. Vione, V. Maurino, C. Minero, D. Borghesi, M. Lucchiari, and E. Pelizzetti, "New processes in the environmental chemistry of nitrite. 2. The role of hydrogen peroxide," Environmental Science and Technology, vol. 37, no. 20, pp. 4635–4641, 2003, doi: 10.1021/es0300259.
[3] S. Goldstein, J. Lind, and G. Merényi, "Chemistry of peroxynitrites as compared to peroxynitrates," Chemical Reviews, vol. 105, no. 6, pp. 2457–2470, 2005, doi: 10.1021/cr0307087.
[4] R. Radi, "Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine," Proceedings of the National Academy of Sciences of the United States of America, vol. 115, no. 23, pp. 5839–5848, 2018, doi: 10.1073/pnas.1804932115.
[5] L. Zhu, C. Gunn, and J. S. Beckman, "Bactericidal activity of peroxynitrite," Archives of Biochemistry and Biophysics, vol. 298, no. 2, pp. 452–457, 1992, doi: 10.1016/0003-9861(92)90434-X.
[6] S. Goldstein, G. Czapski, J. Lind, and G. Merényi, "Carbonate radical ion is the only observable intermediate in the reaction of peroxynitrite with CO2," Chemical Research in Toxicology, vol. 14, no. 9, pp. 1273–1276, 2001, doi: 10.1021/tx0100845.