Sporosan® SD*
Sporosan® SD is a ready-to-use surface disinfectant based on peroxynitrous acid.
*Note! Sporosan® SD is currently in the approval phase and is expected to be available from the end of 2026.

Product Features
Note: Conformity with the Medical Device Regulation (MDR, EU 2017/745) is not yet available. The following product features are aimed for.
Product name: | Sporosan® SD |
Product type: | 2-component disinfectant (ready-to-use) |
Medical device class: | IIB (aimed at) |
Intended use: | Surface disinfection of medical devices |
Active substance: | Peroxynitrous acid (ONOOH)generated from hydrogen peroxide and nitrite as well as isopropanol |
Compatibility: | currently under test (desired compatibility: at least identical to isopropanol) |
Container: | Spray bottle approx. 400 mL |
Shelf life: | 24 months |
Casual temperature: | 0 to 25 °C |
Spectrum of effects: | bactericidal, fungicidal, levurocidal, virucidal, sporicidal and mycobactericidal |
Exposure time: | 60 seconds (4-field test) |
Efficacy
The product Sporosan® SD is currently under development. Currently, only exemplary efficacy data are available. However, these are so convincing that we are very confident in achieving our effectiveness targets.
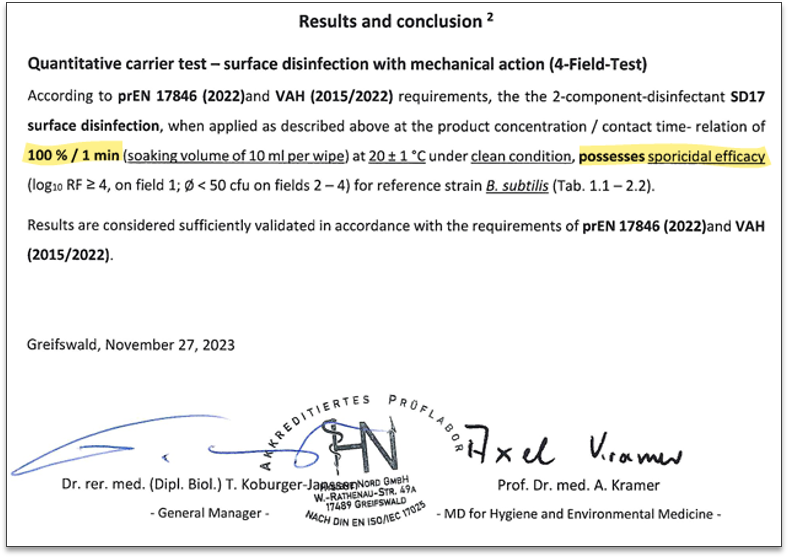
An example summary of the test report of a 4-field test in the accredited test laboratory Hygiene Nord GmbH is shown below. Sporicidal efficacy against Bacillus subtilis spores is achieved within 60 seconds.